Abstract
Introduction: Risk stratification in pediatric acute myeloid leukemia (pAML) is required to determine length and intensity of consolidative therapy. The present scheme employed by COG is complex, relying on numerous upfront cytogenetic and molecular assays along with induction response assessment by flow cytometry to determine final risk status. lncRNAs expression has been reported as a predictive marker in adult AML but has not been comprehensively evaluated in the pediatric population.
Methods: We assayed 68 normal bone marrow (NBM) specimens, 1298 de novo AML cases from Children's Oncology Group (COG) studies and 96 adult AML cases from SWOG Cancer Research Network trials by RNA-seq to enumerate annotated lncRNAs. After updating all pAML cases to modern risk criteria, data were split by blocked randomization by key molecular groups into training, two pediatric validation (60, 20, and 20% of cases, respectively) and an adult validation set. Differentially upregulated lncRNA identified in the training set compared to NBM controls were used to establish a regularized Cox proportional hazards model of EFS; model coefficients from significant lncRNA features were used to calculate a 37-gene lncScore. Survival associations between lncScore and outcomes were tested by Kaplan Meier estimates and the log-rank test. Predictive performance was assessed by concordance testing.
Results: We identified 1346 differentially expressed lncRNA transcripts in comparison of training set pAML to NBM; 647 were upregulated, among which 37 showed a significant survival association. lncScores calculated as the weighted sum of expression by application of these model coefficients to expression data showed nearly equal numbers of patients with positive and negative values in the training cohort (range -1.24 to +1.31), motivating dichotomizing cases by positive or negative lncScore for further analysis.
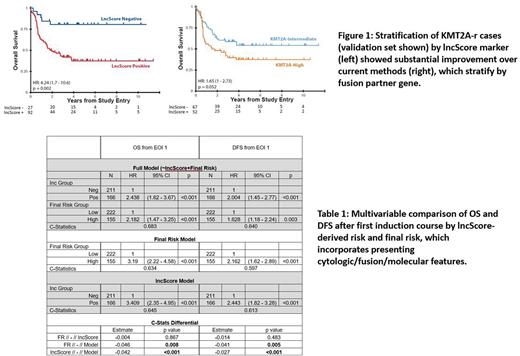
Training set cases with positive lncScores had 5-year EFS and OS rates of 26.7 and 42.7% compared to 56.9 and 76.3% with negative lncScores (hazard ratio, HR, 2.48 and 3.16, p<0.001). Pediatric validation cohorts and an adult AML group yielded comparable results both in lncScore distribution as well as magnitude and significance of outcome effects (pediatric OS HR = 2.87 and 3 respectively, p < 0.001 and EFS HR = 2.38 and 2.36, p < 0.001; adult OS HR=3.21, p < 0.001, EFS HR=2.36, p < 0.001). lncScore remained independently prognostic in multivariable models of pediatric cases including key factors in pre-treatment risk (training set HR 2.07, 1.84, and 1.82 for OS, EFS, and RR from EOI1, all p < 0.001). Concordance statistics exceeded 66% for each survival metric (OS: 0.7, EFS 0.667, RR 0.679). Comparable results were observed in the combined pediatric validation group, (HR OS: 1.75, P=0.001, C-stat 0.672; EFS: 1.56, P=0.001, C-stat 0.671; RR: 1.67, P=0.004, C-stat 0.689). Estimates of the concordance difference between sub-models containing traditional classification compared to lncScore showed negligible differences (p range 0.15 - 0.51) suggesting that the lncScore provides comparable accuracy to current pre-therapy classification metrics. Subgroup analysis suggested that lncScores provide additional outcome information in heterogenous subgroups currently classified as indeterminate risk, such as KMT2A-rearranged (Figure 1).
To test whether lncScores could replace or augment a modern stratification scheme, we compared lncScore to final risk (which includes traditional cytogenetic/molecular and induction response). Point estimates for HR were slightly higher by lncScore than final risk, with all CIs showing substantial overlap. Comparison of single term sub-models in both training and validation groups showed similar trends, slightly favoring lncScore, but with non-significant concordance differential estimates. (Validation set HR for OS 3.41 vs 3.19, C-index diff -0.004, p=0.867, Validation set DFS HR 2.44 vs 2.16, C-index diff -0.014, p=0.483). As with initial risk features, a full model containing final risk and lncScore outperformed either alone, with significant p-values in both possible comparisons (Validation p-value range 0.008 - <0.001, Table 1).
Conclusion: Inclusion of the lncScore enhances predictive power of traditional stratification in pAML with potential, as a single assay, to replace these complex stratification schemes with comparable predictive accuracy.
Disclosures
Farrar:Novartis: Other: provision of study materials, medical writing, Research Funding. Othus:Merck: Consultancy; Celgene: Consultancy; Biosight: Consultancy; Glycomimetics: Consultancy; Daiichi Sankyo.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal